
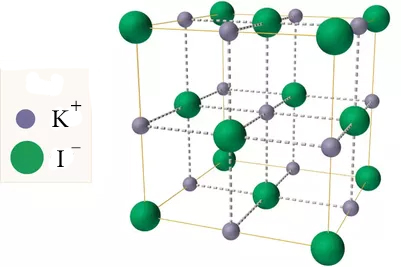
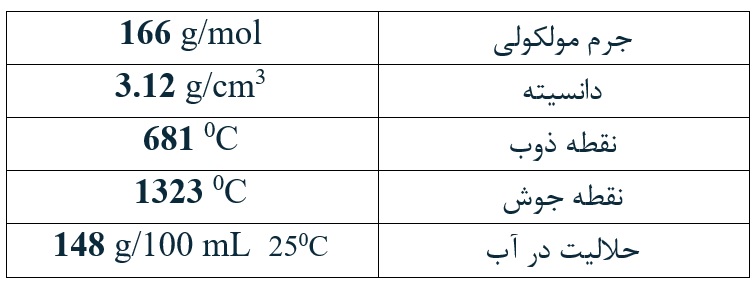
Potassium Iodide with chemical formula of KI [CAS No. 7681-11-0], which crystallizes in a cubic structure, is a white powder that is considered as one of the important sources of iodine and is necessary to produce thyroid hormones. Chemically, iodates are considered among oxidizing compounds and get decomposed at 560 degrees Celsius.

– Iodization of salt :
Potassium iodide is used to iodize the salt in North America and some European countries.
Medication :
– Usage in some iodine-based disinfectants
– Preparation of potassium iodide tablets to prevent absorption of radioactive iodine radiations in nuclear centers.
Other Applications :
– In laboratory analyses in iodometric titrations
– Synthesis of aryl iodide, which is used in the syntheses of organic compounds.
Net Weight : 25 kg
The packaging of all the company’s products is in metallized bags that include 3 layers of polyester, aluminum, and polyethylene.

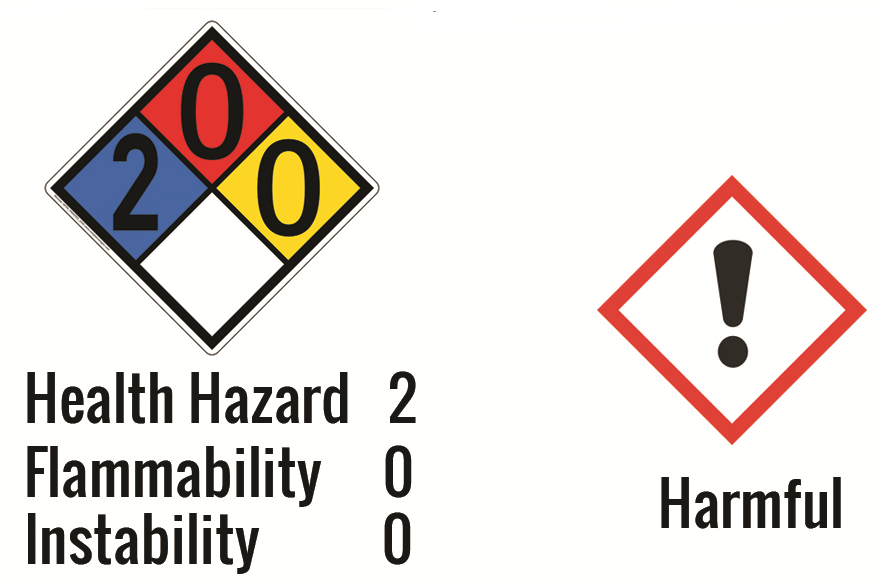
Safety and Storage:
Potassium iodide may react strongly with oxidizing substances such as chlorates, bromates, and perchloric acid and cause the formation of molecular iodine. Avoid storing this material next to the above compounds and store it in a dry environment away from direct light or heat. The direct light can cause yellowing of potassium iodide, which is due to the formation of molecular iodine.
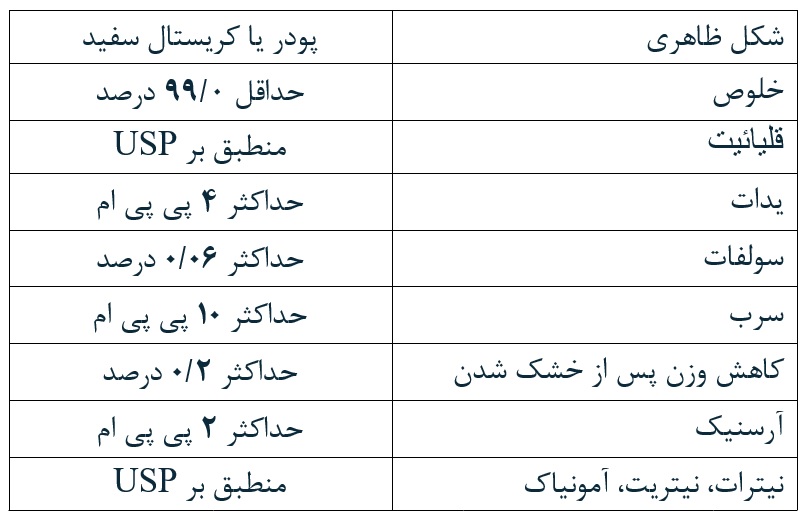
Aghaltin Shimi Cooperative Company was established in 2010 aiming at producing potassium iodide and potassium iodate. Due to the lack of production of these products inside the country, the company decided to meet the domestic needs by producing potassium iodide and potassium iodate at a high-quality level and in accordance with the standards known for the food and pharmaceutical industries.

Production Health License Number : 45/12047

Iran's National Standard Badge

Production License of Iran Veterinary Organization
© All rights are reserved and belong to Aghaltin Shimi Company.